Recently I had an article published along with my colleagues Michael A. Fallucco, MD, FACS,† and Jeffrey E. Janis, MD, FACS‡ on Supraorbital Rim Syndrome: Definition, Surgical Treatment, and Outcomes for Frontal Headache. We wanted to share it with you so we have reprinted it in its entirety here for you.
Migraine headaches directly affect 11% or more of the adult population (almost 35 million Americans).1There are significant direct implications to our healthcare and social systems related to the treatment of these migraine patients and indirect effects because of impaired work performance, detrimental family consequences, social interactions, and quality of life. As far as we have come in defining this symptom complex, the debate continues regarding the true origin. Understanding the origin of migraine headache pain is important to guide acute and preventative treatment strategies as recommended by the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society.2
The peripheral trigger point theory of migraine headaches has gained much support.3 However, a common argument from the centrally triggered theorists is that there are no consistent causative peripheral pathologies.4 In the setting of frontal migraines, we feel the activation of peripheral nociceptors by a nerve compression localized to the supraorbital rim, and involving the branches of the frontal nerve and zygomaticotemporal nerve (ZTN) provides a consistent cause.
The identification of focal peripheral nerve compression sites as a frontal migraine generator adds an option to the stratified care model for migraine treatment.5,6 Current medical treatment options are incomplete for those patients whose supraorbital rim anatomy sensitizes them to migraine headaches. This stimulus originates from the supraorbital nerve (SON), supratrochlear nerve (STN), and ZTN as they exit the orbit.7,8
Peripheral nerve decompression for headaches is not a novel idea. Decompression of the greater occipital nerve was first reported in the neurosurgical literature in the 1960s. In the past decade, several different groups9–13 have described success when performing peripheral nerve decompression to treat headaches. As we know from upper and lower extremity peripheral nerve compression syndromes, there can be multiple sites of potential compression along the same nerve.14,15 The same concept applies to head and neck peripheral nerve anatomy as we demonstrated in cadaver studies for the SON and STN, establishing the more proximal compression to the glabellar myofascial unit.16
We propose the diagnosis of a supraorbital rim syndrome (SORS) as a peripheral nerve compression syndrome contributing to frontal-triggered migraine and headache pain and disability. This article reviews the proximal compression sites of the frontal nerve divisions at the bony supraorbital rim in addition to the glabellar myofascial unit and the contribution of the ZTN to this pain syndrome. Furthermore, we add strategies to facilitate diagnosis and evidence to support the surgical treatment of SORS patients using the stratified care guidelines set forth by the US Headache Consortium.
METHODS
A retrospective review of 276 patients, who underwent nerve decompression/neurectomy procedures for frontal or occipital headache by a single surgeon (R.R.H.), was performed. All surgeries were performed in outpatient setting between 2008 and 2014. Of the 276 patients, treatment of 96 patients included frontal or periorbital deactivation or neuroma resection. This study is an examination of the pure frontal deactivation population of 45 patients.
Diagnosis of SORS
Diagnosis of SORS was based on history of pain emanating from this region, physical examination with tenderness to palpation, and response to diagnostic blocks, demonstrating significant relief of symptoms. Baseline preinjection discomfort was assessed using a standard visual analogue scale. Injections were performed in a step-wise manner with a combination of 1% lidocaine and 0.25% Marcaine17,18 when a patient presented with active pain. Injection of epinephrine-containing solutions was not included in the diagnostic process. Blockade of the SON/STN on the side with the most consistent pain was performed using a 30-gauge needle to inject 0.5 mL at the orbital rim and 0.5 mL within the myofascial component surrounding the nerve. To address the ZTN, 2 mL was injected deep into the temporal fascia to avoid dispersion to the frontal branch of the facial nerve. If needed, a block of the contralateral SON, STN, and ZTN was performed. We have found that those who experience an immediate near total or total relief of symptoms are excellent candidates for surgery.
Anatomy of Frontal Nerve Proximal Compression Sites
The frontal nerve (first division of the trigeminal nerve) is the largest of 3 named branches from the ophthalmic nerve as it enters the posterior orbit at the superior orbital fissure. From the posterior orbit, the frontal nerve begins its intraconal pathway between the levator palpebrae superioris and the periosteum. Along its intraconal route, the frontal nerve branches into the SON and STN.
The SON anatomical variations along its intraconal path to its frontal exit point on the rim account for various potential compression sites. The periosteum provides the first site of compression during the intraconal/frontal exit transition (Fig. (Fig.1).1). If a bony foramen provides the SON frontal exit (27%), this point is a definite closed, nonexpanding site of potential compression (Fig. (Fig.2).2). If a notch provides the SON frontal exit (73%), there can be 1 of 4 variants of fascial bands that complete a carpal tunnel-like ring around the SON (Fig. (Fig.3).3). The SON may branch into the superficial (medial) and deep (lateral) division either proximal or distal to the supraorbital exit, thus the intraconal branching pattern can account for multiple compression sites.
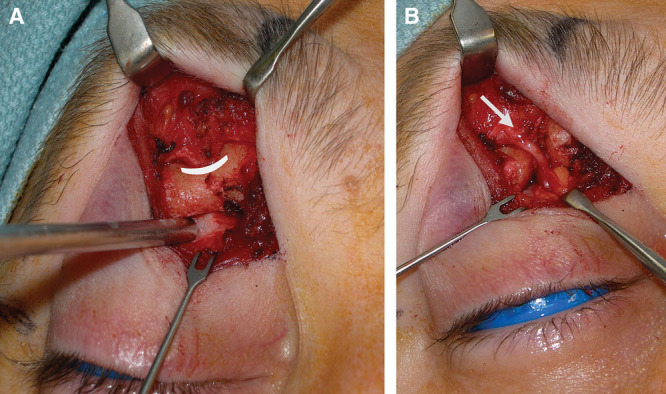
A, SON neurovascular bundle first compression point within a dense periosteal sleeve (pointed out with suction tip) as the SON transitions from its intraconal pathway and exits onto the forehead through a bony foramen. B, Decompressed supraorbital foramen and fascial sleeve (corrugator myofascial component still intact).
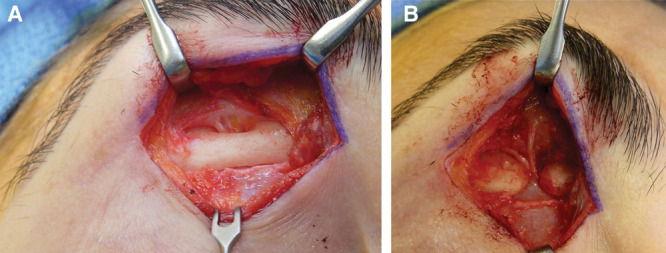
A, Supraorbital nerve compression site at bony foramen with subperiosteal exposure. B, Complete foraminotomy is completed, as well as coagulation of the supraorbital artery and vein. Myofascial release at corrugator and periosteal sleeve release are also accomplished.
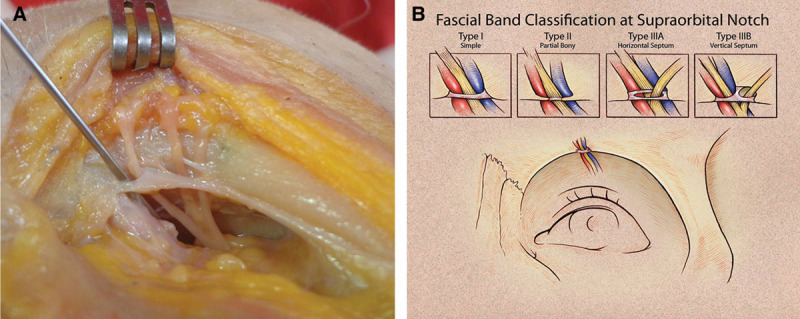
Cadaver demonstration (A) of common IIIA fascial band variant. B, Classification for the SON morphology as it exits the supraorbital rim. Knowledge of these potential compression sites proximal to the glabellar myofascial complex enables complete nerve decompression. Supporting decompression at the orbital rim even when true foramen is not present. Note the separate fascial band exit site for supratrochlear nerve along orbital rim. Reprinted with permission from Fallucco M, Janis JE, Hagan RR. The anatomical morphology of the supraorbital notch: clinical relevance to the surgical treatment of migraine headaches. Plast Reconstr Surg. 2012;130:1227–1233. Promotional and commercial use of the material in print, digital, or mobile device format is prohibited without the permission from the publisher Wolters Kluwer Health (a href=”mailto:dev@null” data-email=”a href=”/>moc.rewulksretlow@snoissimrephtlaeh</a>” class=”oemail” style=”unicode-bidi: bidi-override; direction: rtl; white-space: nowrap; color: rgb(100, 42, 143);”>moc.rewulksretlow@snoissimrephtlaeh).
The proximal STN potential compression site is more consistent as it is held in most cases by a periosteal band along the supraorbital rim (76%). More rarely, there is a true bony foramen for STN exit onto the forehead (18%) (Fig. (Fig.44).19,20 Fig. Fig.55 shows a decompressed SON and STN.
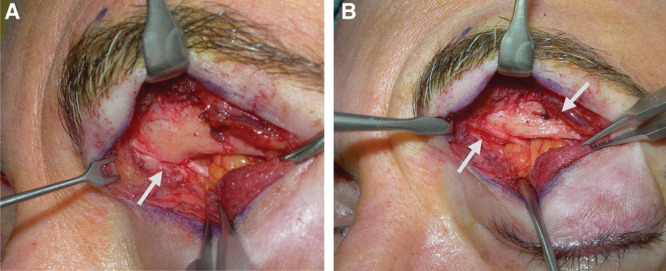
A, Supratrochlear nerve branching within the orbital and entering the supraorbital rim through a bony foramen. B, A STN foraminotomy was performed allowing nerve decompression of this proximal compression site that is proximal to the glabellar myofascial complex. The second arrow shows the SON foramen.
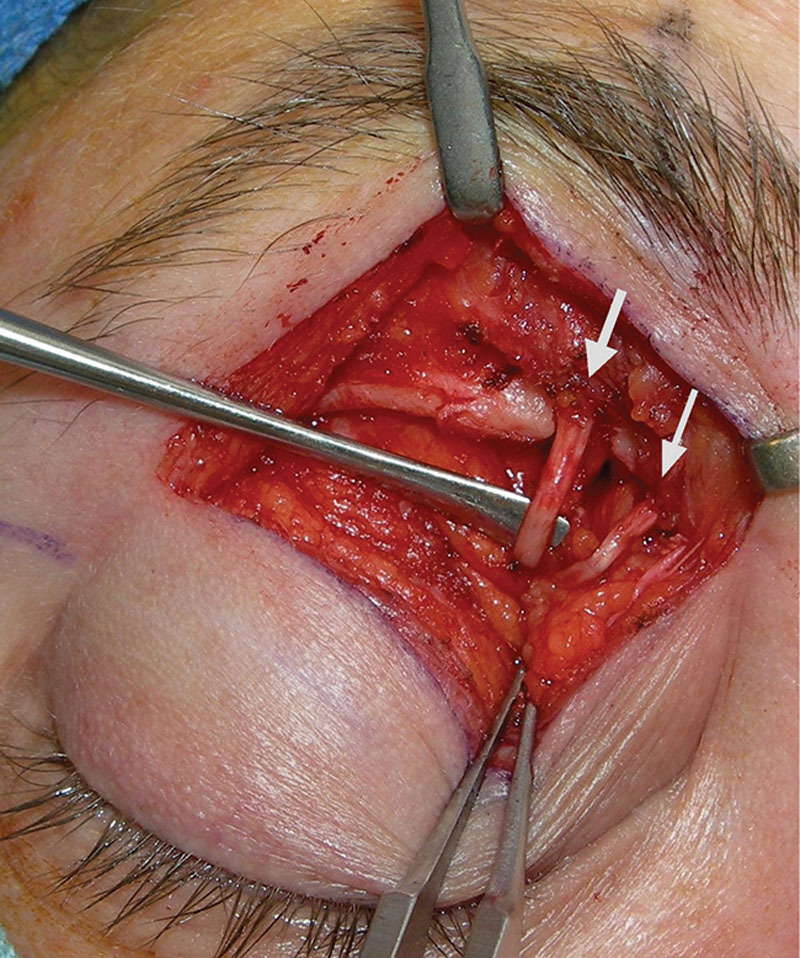
Example of supraorbital and supratrochlear decompression at the orbital rim. Note the quality changes of the nerves at the compression levels.
Surgical Method
All patients included in this study underwent direct, transpalpebral approach and decompression of the SON and STN at the orbital rim and corrugator myofascial sleeve. In addition, zygomaticotemporal neurectomy was performed through a direct approach in all subjects. SON, STN, and ZTN were identified in every case, and possible compressive etiologies were identified, documented, and released.
The standard transpalpebral incision is designed along the upper component of a blepharoplasty incision. In some cases, removal of redundant skin was performed in addition to nerve decompression. Simple direct incisions, without skin excision, are shown in Fig. Fig.6.6. The dissection continues to the decussation of the orbicularis and corrugator where the muscle is divided, exposing the rim. Working from lateral to medial, starting at the lateral limbus line, a subperiosteal dissection along the bony orbital rim is performed; staying right on the rim will protect the lateral SON branch. The SON foramen or notch is first identified followed by the identification of STN rim morphology. Intraconal nerve inspection is used when high or aberrant branches are identified. Although infrequent, the direct approach allows for this inspection easily. A supraorbital foraminotomy is performed, if present, with a small rongeur (Fig. (Fig.7).7). The thickened periosteal sleeve is removed, and the bundle is mobilized. The artery and vein are selectively coagulated with bipolar cautery. If a SON notch is identified, the tight ligament is removed along with partial bony resection. The myofascial unit is best addressed using a freer to identify the fascial sleeve through the muscle, releasing the corrugator muscle fibers superficial to the nerve. The freer is also placed alongside of the lateral (deep) branch of the SON, and its dense attachments are released on the lateral forehead near the temporal fusion line.
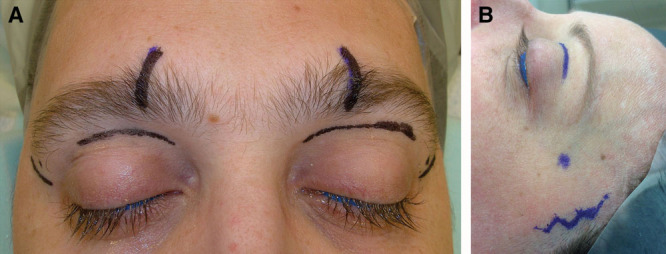
A, Presurgical markings for a direct transpalpebral approach to the supraorbital and supratrochlear nerves (vertical marks within the eyebrow correspond to the location of the supraorbital notch/foramen) with direct crow’s feet incision to access the zygomaticotemporal nerve. B, Anterior hairline or temporal incision for younger patients without periorbital rhytides.
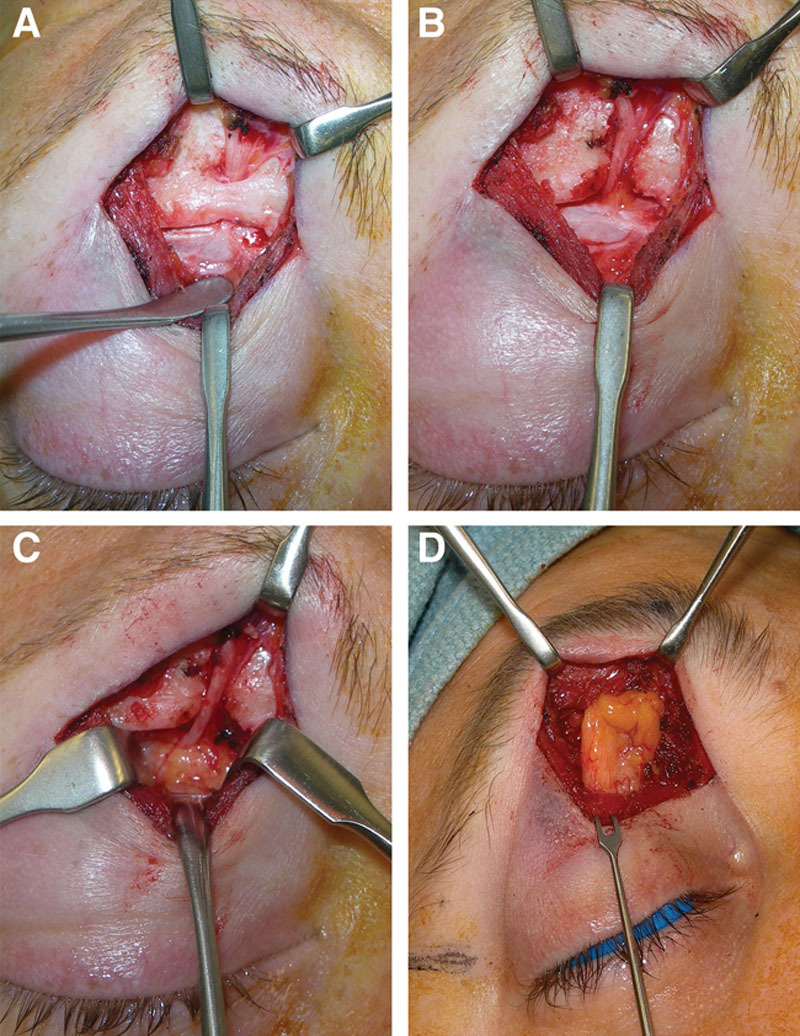
A, SON decompression sequence via direct transpalpebral incision. Dissection is carried down to the supraorbital rim lateral to the SON via the decussation between the orbicularis oculi and corrugator supercilii muscle fibers. B, The periosteum of the orbital rim is reflected allowing intraconal view of the orbital roof for full visualization of the SON and STN as the nerve divide from the frontal branch of the trigeminal nerve. C, A foraminotomy is performed with a rongeur, vessels are coagulated, the nerve is mobilized, and the periosteal sleeve is released allowing herniation of the orbital fat. D, The local, vascularized, medial fat pad is mobilized on its pedicle and transposed into the orbital rim defect, allowing healthy vascularized tissue to surround the decompressed SON.
The STN is addressed in a similar fashion. The STN most consistently exits below a dense broad periosteal or fascial band but may also enter onto the rim through a bony foramen.19,20 Many times, it is easier to find it intraconally and follow it to the orbital rim exit. Careful attention should be given to the trochlea when dissecting this nerve. Full decompression should be accomplished at the rim and corrugator muscle. On occasion, if the STN is quite small, a neurectomy proximal to the orbital rim exit is performed with the end buried within the deeper orbit fat.
A local pedicled, fat flap is fashioned from the medial compartment and transposed in to the defect from the corrugator release (Fig. (Fig.7D).7D). This also provides benefit to the nerve as this positions vascularized fat to protect and heal the nerve.
The ZTN incision can be a small extension of the transpalpebral incision into a crow’s feet rhytid or can be a direct incision into the rhytid when no upper lid skin is being excised. An alternative is the temporal scalp incision, which works well in a younger patient who lacks periorbital rhytides and has a forward hairline.21The nerve is located inferior to the sentinel vein (Fig. (Fig.8).8). Once identified, a neurectomy is performed. Often, some of the anterior temporalis fibers are divided as well to look for any duplicate aberrant branches.
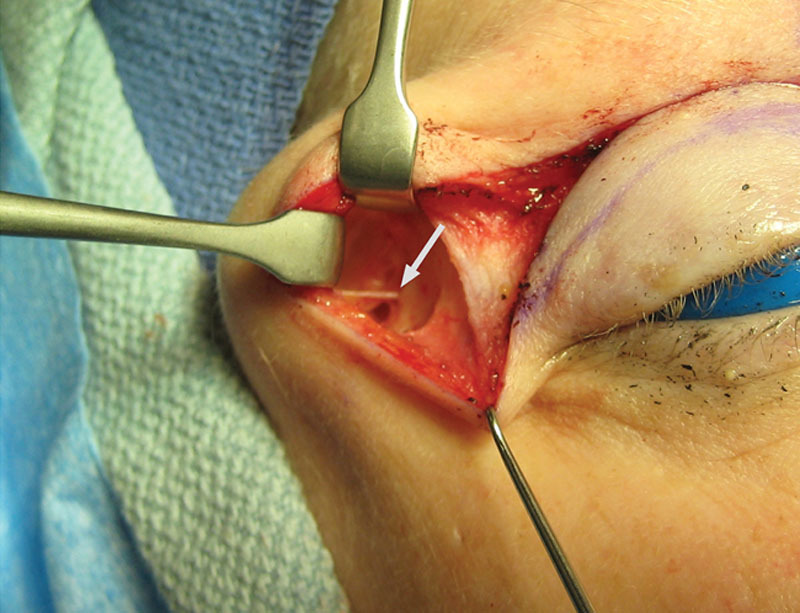
Example of the zygomaticotemporal nerve as it emerges from the deep temporal fascia. Approach can be through crow’s feet incision or as a lateral extension of the transpalpebral incision if upper lid skin excision is planned. Alternatively, a temporal scalp incision can be used in the younger patient with a forward hairline.
Migraine Disability Assessment Questionnaire
All patients filled out a Migraine Disability Assessment Questionnaire (MIDAS)22 at their initial evaluation and on the day of surgery. Postoperative MIDAS scores were collected at 3 and 12 months.
Statistical Analysis
Statistical analysis was performed with a paired t test for preoperative and postoperative MIDAS scores.
RESULTS
A retrospective review of 276 patients who underwent nerve decompression/neurectomy procedures to relieve headache pain was performed by a single surgeon. Within this group, treatment of 96 patients included frontal or periorbital deactivation or neuroma resection. The pure frontal deactivation population of 45 patients was examined in this study. Thirty-four of the procedures were bilateral, and 11 were unilateral. In total, 79 orbits were surgically treated with this technique, involving 237 nerves. The patients were predominantly women with ages ranging from 18 to 77 years, averaging 47 years old.
The average preoperative MIDAS score was 134. Postoperatively, MIDAS scores decreased significantly at 3 months to 25 and remained at 24, when measured at 12 months postoperatively (P < 0.0001 vs baseline; Fig. Fig.9).9). Adverse events were infrequent and included persistent swelling (n = 2 patients; resolved by 6 weeks), hematoma (n = 1 patient; a minor subcutaneous hematoma that resolved on its own), infection/cellulitis (n = 1 patient), and neuroma (n = 1 patient; treated with a short course of amitriptyline); all of these were resolved without further surgery.
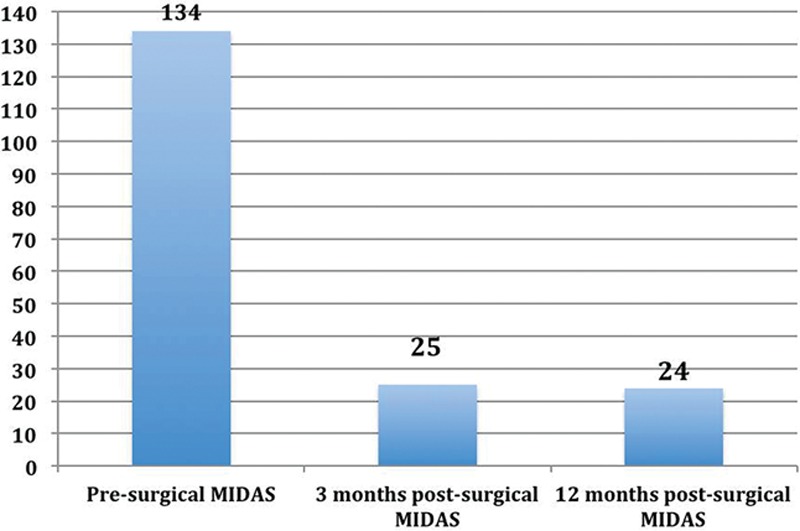
Presurgical and 3- and 12-month postsurgical MIDAS. Statistical analysis was performed with a paired t test for preoperative and postoperative MIDAS scores. After surgical intervention, MIDAS scores decreased significantly at both 3 and 12 months postoperatively (P < 0.0001 vs baseline).
We analyzed at percentage change in the MIDAS and divided these into 4 categories noted in Table Table1.1. Types 1 and 2 equate to true functional life improvements. Type 3 represents intermediate improvement. Type 4 represents less than 50% MIDAS reduction and essentially failure to respond to surgical deactivation.
Table 1.
Percent Improvement of MIDAS Scores at 12 mo Postoperatively
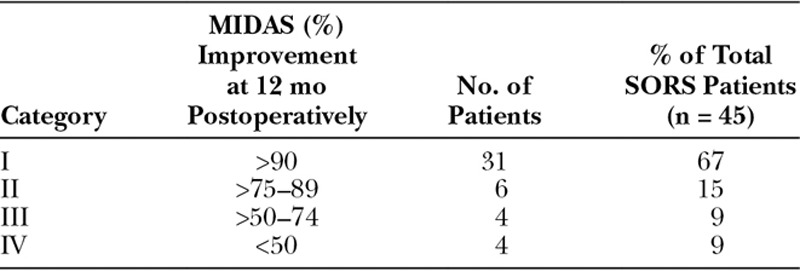
Ten percent of patients reduced their MIDAS score to 0, meaning a 100% reduction. Sixty-seven percent reduced their MIDAS by more than 90%. Eight-two percent of patients decreased their MIDAS by more than 75%. Ninety-one percent of patients decreased their MIDAS by more than 50%. Nine percent of patients had less than 50% reduction in their MIDAS, which we considered failure to respond. Of interest, 1 of the 4 (type 4) failures maintains that they would still have surgery. Three of the 4 type 3 patients maintain that they would still have surgery given some benefit.
DISCUSSION
Compression of the peripheral nerves of the supraorbital rim (SON, STN, and ZTN) can have varying underlying etiologies.23 Decompression of these nerves by addressing muscle, fascia, bone, or vessel can result in significant improvement in headache pain. Decompression of the SON at the supraorbital rim with positive results on patients with frontal pain syndromes has been reported by Sjaastad et al24 in 1999. Sjaastad et al24 correctly identified a “fascial band or bony extension” at the supraorbital notch, which they removed in 5 patients. This article provides additional evidence that decompression of the myofascial sleeve of the SON and STN combined with the more proximal decompression of fascial and/or bony elements at the supraorbital rim represents a more complete decompression.
In both our published cadaver dissections and clinical experience, we have identified critical anatomical points. A true foramen and notch with a band both provide a fixed, nonexpanding bony aperture for supraorbital neurovascular passages and represent a natural compression point. In our clinical experience, this represents the most consistent anatomic compression point. Also, a confluence of periosteum often tightly ensleeves the SON as it transitions from its intraconal pathway to the frontal exit, which when present, should be removed. When a notch is present, there are 4 variations of the fascial band morphology and potential compression. However, whether the fascial band represents an extension of the arcus marginalis or is of its own embryonic origin (such as with a persistent band in radial dysplasia) is unknown. Understanding the variations of horizontal and vertical septa that may be present is important clinically to achieve complete decompression of the nerve. For instance, if the SON branches into its superficial and deep branches proximal to notch exit, horizontal or vertical septa would provide a separate tunnel for each branch. Incomplete decompression of only the fascial band surrounding the medial (superficial) branch will still perpetuate a
from the lateral (deep) branch. Furthermore, we have consistently performed a conservative muscle resection when addressing the myofascial units within the corrugator, releasing only the fibers superficial to the nerve. This approach is supported by how we
in the extremities. For instance, we do not resect the entire pronator muscle when decompressing a proximal median compression.7
The frontal exit of the STN is more consistent as an extension of the arcus marginalis and has been previously demonstrated to have notch and foramen variants as well.19 These findings support the presence of compression at both the orbital rim and the myofascial unit just like the SON, which emphasizes the importance of releasing both sites.
We postulate that variable, asymmetric rim morphologies in the same patient may represent the unilaterality or one-sided dominance in this type of peripherally triggered headache syndrome.
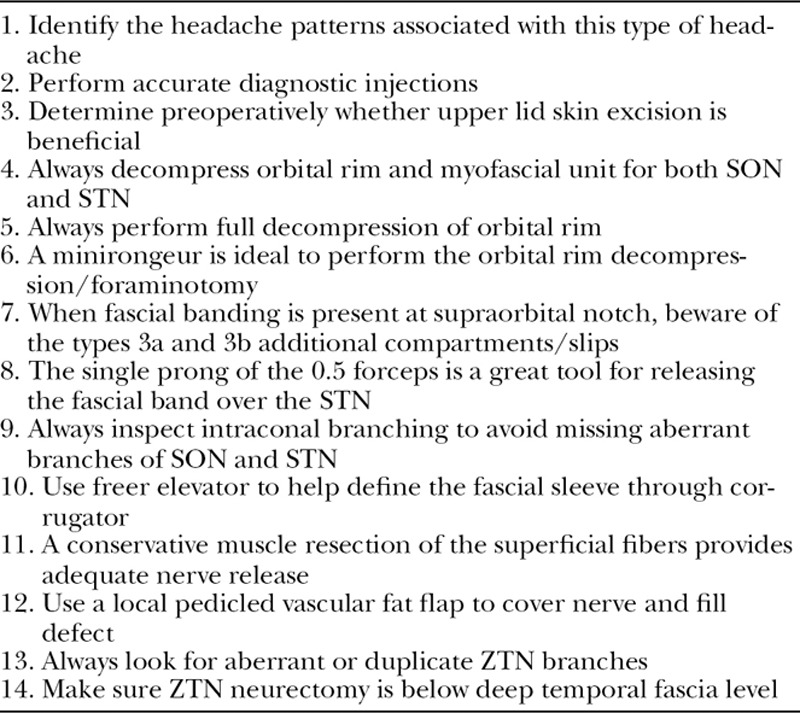
We feel that the transpalpebral approach is optimal given its easily concealed incision and gives adequate, direct exposure to the nerve at the intraconal space, orbital rim, and the myofascial levels (Table (Table2).2). Complete evaluation of the supraorbital rim anatomy and notch/foramen morphology, as well as the release of the fascial band or foraminotomy, often requires maneuvers that, in most hands, would be more challenging via the endoscopic approach. Also, it is not uncommon for these patients to have significant upper lid excess that contributes to a hypercontracted/dynamic forehead and brow musculature, which secondarily can potentially cause unwanted traction on the already irritated/sensitized nerve. The direct approach allows us to easily incorporate a traditional upper lid skin excision to address this. Pearls for the surgical treatment of SORS are shown in Table Table33.
Table 2.
Benefits of Direct or Transpalpebral Approach for the Nerves Involved in SORS

Table 3.
Pearls for Surgical Treatment of SORS
The MIDAS questionnaire has been shown to correlate with both physician’s assessment of treatment need and outcomes of treatment.22 The MIDAS questionnaire categorizes patients into 4 grades based on their illness severity. With obvious selection bias as a surgical tertiary referral, our patients all fall into the severe disability or MIDAS grade 4. All of our patients to date have a MIDAS score that is well above the severe disability grade. These grade 4 patients represent a subset of migraine headache pain patients who have received an accurate diagnosis but are not receiving suitable therapy through medical or alternative medicine treatment arms.25 It is this subset of patients that surgical intervention should be considered and incorporated into individualized management and not thought of as a last resort.
We acknowledge that the ultimate patient sample size in this study is limited. However, this subpopulation represents a pure population of patients who underwent this specific surgical technique within our larger, comprehensive headache surgery experience and is not confounded by the performance of alternative skin incisions, surgical approaches, and treatment of other trigger sites. Furthermore, it does however represent a larger number than other studies for these specific trigger sites. Nonetheless, the early experience with this technique is significant and sets the stage for continued studies on this topic.
CONCLUSIONS
Understanding that head and neck nerve compressions share conceptual similarities with extremity compression syndromes allows us to apply the concept of multiple anatomical points of compression to the pathology of migraine triggers or chronic headaches. In those patients whose disability is related to frontal pain, we offer insight into consistent anatomical points at the supraorbital rim that, if decompressed, has shown to offer significant relief to these patients. Cadaver and clinical experience points to a fixed (bone/ligament) and dynamic (myofascial) compression site at the supraorbital rim, causing a SORS, which is treatable in the outpatient setting. In our population of 45 patients, surgical intervention resulted in significantly decreased MIDAS scores.
ACKNOWLEDGMENTS
We thank Joanne McAndrews, PhD, for assistance with the preparation of this article.
Footnotes
Disclosures: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.